lethality calculation for autoclave|steam sterilization 2007 pdf : wholesale The purpose of this Fedegari Technical Note, firstly distributed in 1988 and perseveringly revised, is to clarify the nature of F0 and its related parameters (D, z, PNSU/SAL), and to explain their . BMT USA is a leading supplier of cGMP steam sterilizers and life science solutions for pharmaceutical, biotech, and laboratory applications.
{plog:ftitle_list}
In conclusione, il legno autoclavato combina le qualità naturali del legno con i benefici del trattamento in autoclave, offrendo un materiale resistente, stabile e esteticamente gradevole. Le sue caratteristiche uniche lo rendono una scelta .
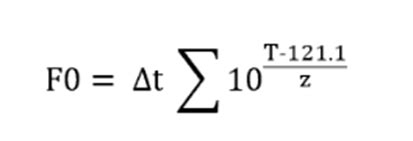
Calculation of D - Value, Z - Value and F0 - Value for sterilization in autoclave used for microbiology analysis as per USP and BP.For example, if a material has a bioburden of 540 cfu then to reduce the microbial populatio.
The purpose of this Fedegari Technical Note, firstly distributed in 1988 and perseveringly revised, is to clarify the nature of F0 and its related parameters (D, z, PNSU/SAL), and to explain their . Data such as these can be converted to equivalent time for D-value, SLR, and SAL calculations using the mathematical model described below. The technique is based on lethality rate (L R), which can be expressed either as a rate function with units of Dlog N per minute at specified conditions or as the reciprocal of the D-value. Autoclave efficacy is highly dependent on time, temperature, and pressure, and these parameters can be manipulated and optimized to create specific sterilization cycles for each application. To help your facility get the .This number adds up the lethality values for each time interval and calculates an approximation of the area under the lethal rate curve. This value will be referred to as the “computed cumulative F value” or the “cumulative process lethality”. In the given example, the calculation results in an equivalent lethality at 144 F of 76.90 .
steam sterilization formula f0
steam sterilization 2007 pdf
Calculator for determining the lethality (F, value) of a thermal process using the trapezoid and Simpson's rules. This calculator has been designed to work with pasted time and temperature values from a data logger or individual keyboard inputs. It is unique in that users can compare F values obtained by integrating time / lethality values .
F0 Target: the target of lethality result that you want to obtain from your process expressed in minutes and seconds; Start Let. Cal. (°C) (Start Lethality Calculation): the minimum temperature from which to start the Lethality calculation. All contributions to the final result recorded with temperatures lower than the one set in the field .Laboratory Testing Services for Medical Devices in Rhode Island
f0 value for steam sterilization
What this definition suggests is that overkill requires a 12-D process, which equates to lethality sufficient to deliver a 12 × D 121 lethality level. This is not a lethality standard at all, however, because it inappropriately links the process lethality requirement to the characteristics of a specific biological indicator (BI). The F0 value is defined as the thermal lethality time required to eliminate all microorganisms present in a sample, by exposing them to a temperature of 121.1ºC and it is expressed in minutes. To calculate the F0 value, average the temperature of each probe during the sterile hold and then average the different temperatures to get one single .Lethality Overview Input parameter Start Date : 03-04-2008 Ref. Temperature: 121,10 °C Min. Temperature : 91,10 °C Z-Value: 10 °C D-Value: . Steam Autoclave UTC offset 01:00:00 Validation Manager Vessel: Session Text: Validation 26-11-2013 12:52:20 26-11-2013 12:52:20 Sterilization Validation Sterilizer Pharma Medical Autoclave is used for sterilization of various articles in microbiology laboratory as well in sterile manufacturing. This article has procedure for autoclave validation including steam penetration, heat distribution and penetration, bio-challenge study, estimation of F0 value and acceptance criteria of steam sterilizer validation in pharmaceutical industry.
Calculation of productive capacity. Calculate online and free of charge your productive capacity per cycle. . The F 0 value is defined as the thermal lethality time required to eliminate all microorganisms present in foods, . Secondly, we have the actual F 0 value, the F 0 value we really achieved in our autoclave thermal processing, .The F 0, at a particular temperature other than 121, is the time (in minutes) required to provide the lethality equivalent to that provided at 121 for a stated time. Modern autoclaves generally operate with a control system that is significantly more responsive than the steam reduction valve of older units that have been in service for many years.A description of the autoclave process, including pertinent information such as cycle type (e.g., saturated steam, water immersion, and water spray), cycle parameters and performanceH ow do we process these values to obtain a value for F 0 for this heat treatment? There are several ways of implementing the Trapezoid Rule. One way is to create 4 columns in Excel as indicated in Table 2. Table 2. Excel spreadsheet for calculating F 0. Lethality is calculated using equation 1 by entering the formula “=10^((B4-121.1)/10)” (remove the “”) at cell C3 and copying .

Using a Lethality Table to calculate the heat process value F is, unfortunately, a very tedious activity. Therefore both Worked Examples 1 and 2 also outline the application of two (down-loadable .Note: The z-value of the biological indicator for moist heat processes can be a value other than 10 °C (the number used in the F 0 calculations), which can lead to differences in the physically integrated lethality and the directly measured . For this cycle, consider the F0 value setpoint as 30 minutes. Here, the calculation part plays an important role in effectively controlling the sterilization cycle. Note: F0 calculation can either start from Heating 1 or from .
In the past two decades, aseptic processing has been implemented in the food industry to sterilize particulate liquid food mixtures. To ensure that particulates in the liquid receive sufficient heating, mathematical modeling is employed to evaluate the temperature and lethality level in the particles. We developed a model for the thermal processing of liquid foods .eficacy is highly dependent upon actual temperature. For example, if a steam autoclave is running at 120°C for 15 minutes, the theoretical lethality of that cycle is only 82% of a cycle running at 122°C for the same amount of exposure time. Since most laboratory autoclaves do not require temperature to be accurate to better than ±1°C,
Laboratory Testing Services for Medical Devices in Rhode Island

test for hamstring tear
Note that the Z-value has a significant effect on F value, refer to the free download of lethality tables formulated using Z-values of 6°,8°,10° and 12°C. There is also a facility to perform a lethality calculation using an uploaded CSV file. Basic instructions are provided in the help file. The application can be tested using internal data . Any autoclave validation, for either overkill or product-specific load cycles, must demonstrate the delivered lethality to the most difficult locations using biological and physical data. More than being just a “dead or alive” reading, the BI does enumerate delivered lethality when assessed appropriately.
15. Lethality in Dry Heat Sterilization Time Temperature Lethality Rate (min) (0C) min. at 170 0C 5 105 0,0006 10 110 0,0010 15 120 0,0032 20 135 0,0178 25 150 0,1000 30 165 0,5623 35 170 1,0000 40 172 1,2589 45 174 1,5849 50 174 1,5849 55 174 1,5849 60 175 1,7782 65 165 0,5623 70 150 0,1000 75 140 0,0316 80 130 0,0100 85 110 0,0010 90 105 0,0006 – .
test for knee meniscus tear
Note that when Z = 10.0°C and the reference temperature = 121°C we can denote the lethality as F 0.For all other Z-values the symbol to denote lethality is simply F or F T,Z where T = any reference temperature and Z = specific Z-value.. To calculate the total cycle lethality, one must calculate the value for each segment of the cycle curve.H ow do we process these values to obtain a value for F 0 for this heat treatment? There are several ways of implementing the Trapezoid Rule. One way is to create 4 columns in Excel as indicated in Table 2. Table 2. Excel spreadsheet for calculating F 0. Lethality is calculated using equation 1 by entering the formula “=10^((B4-121.1)/10)” (remove the “”) at cell C3 and copying .Advanced Validation Technology. The Kaye Validator AVS (Advanced Validation System) is an all-in-one temperature mapping and thermal validation system that combines precise sensor measurements with all GxP requirements for calibration and traceability to national standards, while generating compliant reports and managing the validated assets and validation equipment.
test for labral tear of shoulder
test for ligament tear
The Touchclave-R range is ideal for installation in laboratories which do not have access to a drain or water supply. Equipped with features and a range of options not normally seen on this type of sterilizer, the Touchclave-R .
lethality calculation for autoclave|steam sterilization 2007 pdf